Ideally a good chromatogram should have the following charecterstics:
1- The peaks should be sharp and symmetric.
2- Peaks should be well separated from one another ( each peak should srtart at the baseline and comes back down to the baseline).
3- The base line should be straight ( with no drifting up or down and no underlying impurities).
4- The base line should be free from background or electric noise. (What is the difference between 3 and 4).
5-The chromatographic run time should be reasonable (15 minutes is ideal).
6-The developed procedure should have good sensitivity.
7-When the analysis is repeated under the same conditions, the chromatogram should be reproducible.
A good chromatogram may look like one of the following examples:
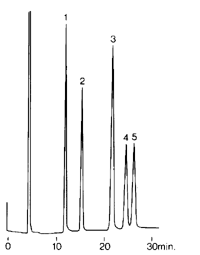
Chromatogram (1)
Chromatogram(4)
Chromatogram (3)
The peaks in all chromatograms are well separated, sharp and they all come back down to the base line. There are no underlying impurity, no drifts and no noise in the base line. The analysis time is reasonable. In chromatogram (2), the analysis time is a little longer than would be concidered optimal but the number of components to be separated are large and the good resolution between the peaks makes up for the slightly longer time of chromatographic analysis. However, obtaining an ideal chromatogram like one of the above is only acheived after an effort. Although knowlege of the nature of the mixture to be analized and experience in chromatography may help us arrive at a good combination of stationary and mobile phase that produces a reasonable chromatogram. Some times highly unacceptable chromatograms like the ones below may be obtained .

In this chromatogram although the chromatographing time is reasonable, peak 2 and 3 are not well resolved. peak 1 seams to have another peak underneath it causing a shouder (lack of symmetry) after the epak. In this chromatogram resolution is very bad. The baseline is drifting upwords. This would not allow for proper calculation of peak area or hight.
In this chromatogram resolution is very bad. The baseline is drifting upwords. This would not allow for proper calculation of peak area or hight.
Some times slight modification of the mobile phase will improve the separation and in other cases the chromatgraphic separation may need a drastic change in the mobile pase or the type of the stationary phase which may even alter the sequence of elution. Study the following examples.
 (B)
(B)The two achromatograms (A) and (B) are of the same mixture of two components. The mobile ohase used to develop chromatogram (A) did not lead to complete separation of the two components. Therefore chaniging the mobile phase lead to much better separation in the above chromatogram(B).
(A)
The same argument holds for the mixture below:

Chromatogram(A)

(B)
Now in this example a very good resolution was obtained using mobile phase (A) changing the mobile phase in (B) only increased the run time. Therefore there was no need to change chromatographic conditions.
 {A} In the above chromatogram (A)although the peaks seam well separated peak 7, 6 are eluted logether. This may indicate that they are very close in polarity.
{A} In the above chromatogram (A)although the peaks seam well separated peak 7, 6 are eluted logether. This may indicate that they are very close in polarity.{B} In the second chromatogram a different mixture of solvents was used as a mobile phase with completely different polarity. The result was a much better chromatogram, well resoved peaks but, on the other hand the order of elution of the peaks was changed.

...........II........................III
......I
IV





3 comments:
About the mixtures..
For the first one, there is noise in the baseline "could be due to impurities in the mobile phase"
2nd case: baseline drift "downwards"
and 3rd case: if I am not mistaken, I think the peaks show tailing
Radwa when looking at the two chromatograms given the number I, if you have to choose between the two chromatograms, both show good resolution although the lower one shows some baseline noise but non interferes with the peaks. So the only advantage of the top chromatogram is that the analysis time is shorter. The same goes for the difference between chromatograms II and III. Chromatogram IV is ideal. What seems to be tailing is very insignificant.
Post a Comment